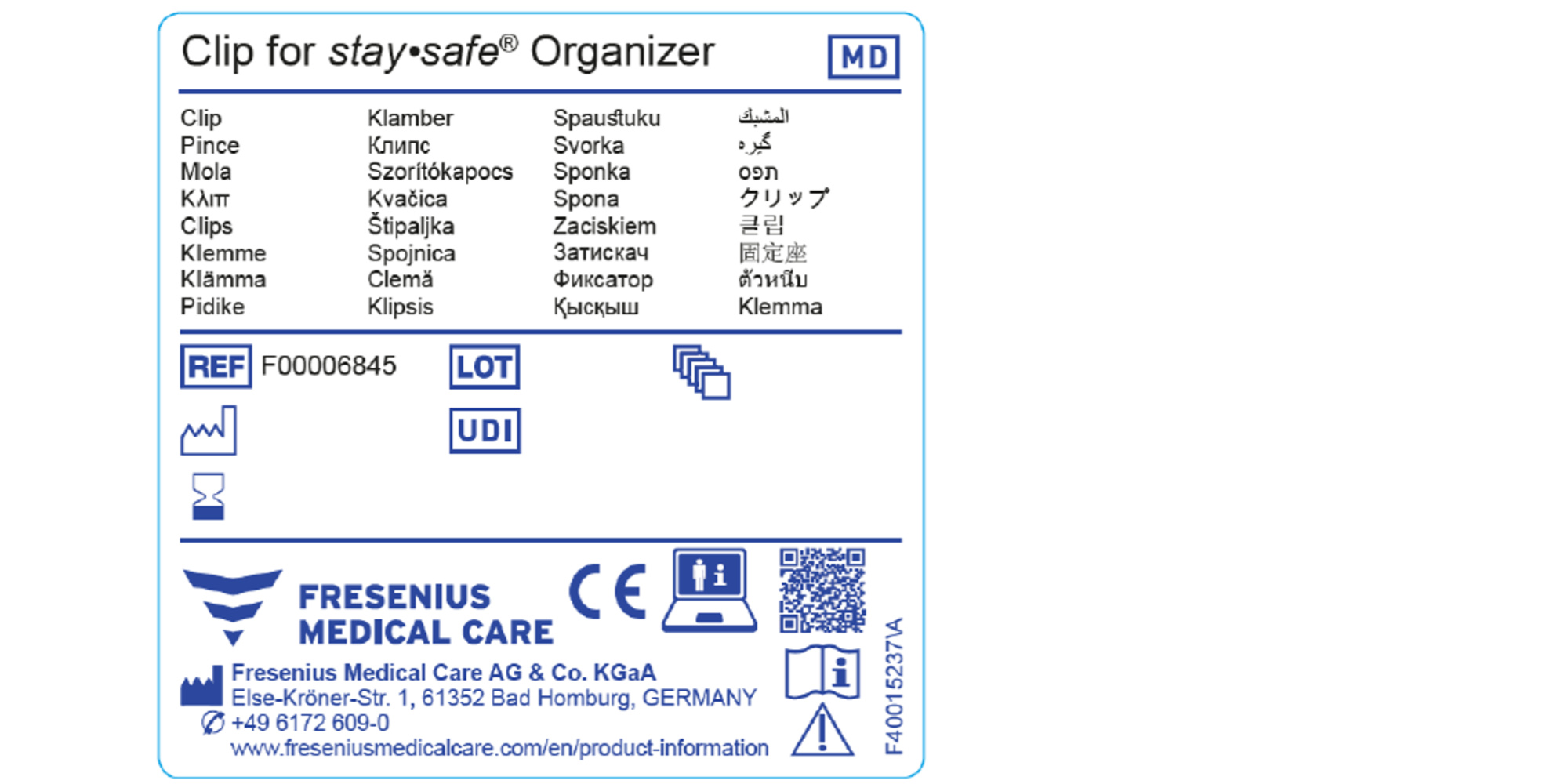
Medical Devices Labels . the general labeling requirements for medical devices are contained in 21 cfr part 801. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. medical device symbols are essential for conveying vital information on medical devices. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to.
from mungfali.com
the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. medical device symbols are essential for conveying vital information on medical devices. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. the general labeling requirements for medical devices are contained in 21 cfr part 801.
Medical Device Labeling Symbols
Medical Devices Labels labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. medical device symbols are essential for conveying vital information on medical devices. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. the general labeling requirements for medical devices are contained in 21 cfr part 801.
From www.flexo-graphics.com
Medical Device Labeling Archives FlexoGraphics Medical Devices Labels product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask. Medical Devices Labels.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Devices Labels labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. medical device symbols are essential for conveying vital information on medical. Medical Devices Labels.
From old.sermitsiaq.ag
Medical Device Label Template Medical Devices Labels product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. the general labeling requirements for medical devices are contained in 21 cfr part 801. labelling serves to identify a device and its manufacturer, and to communicate information on safety,. Medical Devices Labels.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Medical Devices Labels the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. the general labeling requirements for medical devices are contained in 21 cfr part 801. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. medical device symbols are essential for conveying. Medical Devices Labels.
From coastlabel.com
Medical Device Labeling Medical Equipment Labels Coast Label Medical Devices Labels labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask. Medical Devices Labels.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Devices Labels the general labeling requirements for medical devices are contained in 21 cfr part 801. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device. Medical Devices Labels.
From clin-r.com
Labels for Medical Devices Clin R Medical Devices Labels the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. labelling serves to identify a device and its manufacturer, and to communicate. Medical Devices Labels.
From mavink.com
Medical Device Labeling Symbols Medical Devices Labels labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. the general labeling requirements for medical devices are contained in 21 cfr part 801. medical device symbols are essential for conveying. Medical Devices Labels.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Devices Labels the general labeling requirements for medical devices are contained in 21 cfr part 801. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. medical device symbols are essential for conveying vital information on medical devices. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for. Medical Devices Labels.
From mavink.com
Medical Device Labeling Symbols Medical Devices Labels the general labeling requirements for medical devices are contained in 21 cfr part 801. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. medical device symbols are essential for conveying vital information on medical devices. labelling serves to identify a device and its manufacturer, and to communicate information. Medical Devices Labels.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Medical Devices Labels labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what. Medical Devices Labels.
From clin-r.com
Labels for Medical Devices Clin R Medical Devices Labels medical device symbols are essential for conveying vital information on medical devices. the general labeling requirements for medical devices are contained in 21 cfr part 801. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for. Medical Devices Labels.
From mavink.com
Medical Device Labeling Symbols Medical Devices Labels product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. the general labeling requirements for medical devices are contained in 21 cfr part 801. medical device symbols are essential for conveying vital information on medical devices. the medical. Medical Devices Labels.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Devices Labels the general labeling requirements for medical devices are contained in 21 cfr part 801. medical device symbols are essential for conveying vital information on medical devices. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for. Medical Devices Labels.
From www.kmedhealth.com
Medical Device Label SymbolsI In Packaging Medical Devices Labels the general labeling requirements for medical devices are contained in 21 cfr part 801. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. product labels on medical devices help to. Medical Devices Labels.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Medical Devices Labels medical device symbols are essential for conveying vital information on medical devices. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and. Medical Devices Labels.
From mungfali.com
FDA Medical Device Label Symbols Medical Devices Labels medical device symbols are essential for conveying vital information on medical devices. labelling serves to identify a device and its manufacturer, and to communicate information on safety, use, and performance. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. product labels on medical devices help to educate patients. Medical Devices Labels.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Medical Devices Labels the general labeling requirements for medical devices are contained in 21 cfr part 801. product labels on medical devices help to educate patients about how a device should be used, who should use the device, what risks the device could pose to. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of. Medical Devices Labels.